Abstract
Myelodysplastic syndrome (MDS) is a group of disorders involving hematopoietic dysfunction, with a leukemic transformation characteristic. The risk based therapeutic strategy was well applied in clinical. And for patients with IPSS low or intermediate risk-1, NCCN guideline recommended hematopoietic stimulation therapy, immune suppression therapy (IST), as well as demethylation therapy, along with best supportive care. Eltrombopag (EPAG) was firstly approved as the second line option for immune thrombocytopenia (ITP), and recently it was also investigated for treatment of aplastic anemia and MDS. In MDS, based on available reports, the EPAG was mostly used along with hypomethylating-agent, or as salvage treatment after treatment failure. However, there was study showed that the combination of EPAG to azacitidine (AZA) may worsen platelet recovery, with lower response rate and increased trend of leukemic transformation. The successful EPAG monotherapy for MDS case was report, with complete hematologic remission and blasts elimination.
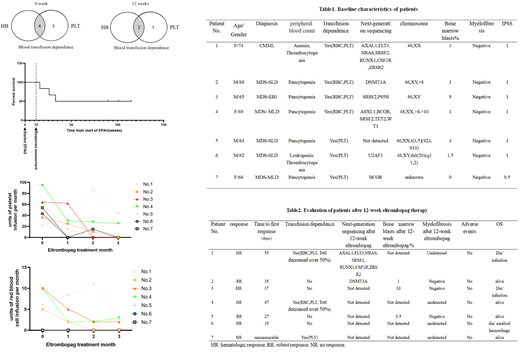
To date, limited study was focus on investigating the benefit of EPAG as initial monotherapy for transfusion dependent (TD) patients with low to intermediate risk-1 MDS, as well as its safety. Herein, we reviewed cases of elderly TD-MDS patients who received EPAG as front-line single treatment in our center, and 7 patients was enrolled from July, 2019 to September, 2020. Patients received EPAG 25mg to a maximum 75mg. The side effects, numbers of transfusion, improvement of peripheral blood and bone-marrow blast were recorded.
The 12weeks overall response rate was 85.7%, with 2 robust response (RR), and 4 hematologic response. In patients got response, 66.7% (4/6) became platelet transfusion independent, with an answering period from 19 days to 55 days. No drug adverse events were observed within 12 week-treatment, and no obvious increased blasts as well as myelofibrosis were observed. 2 patients repeated the next-generation sequencing, and no new mutations were found. After the 12-week treatment, the 2 RR patients were still taking EPAG and got continued complete remission with a median follow up of two years. Two patient (No.3 and No.4) relapsed after EPAG withdrawn and became transfusion depend again. Patient No.1 and No.6 died of infection and cerebral hemorrhage during the follow up.
In summary, EPAG may improve the hematopoiesis of TD-patient with low to intermediate risk-1 MDS, and was well tolerant with a dose of 25mg to 75mg for Asian population.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal